number of valence electrons in aluminum|How to Find the Valence Electrons for Aluminum (Al)? : Tuguegarao The second element in group-13 is aluminum. The valence electron is the total number of electrons in the last orbit. The total number of electrons in the last shell after the electron configuration of aluminumis called the valence electrons of aluminum(Al). . Tingnan ang higit pa Deutsch English Français 日本語 正體字 Play now Posts All past news articles. Halloween Event 2023. October 31, 2023. Liebe Spieler, Nach der Wartung am 26. Oktober 2023 kommen gleich zwei Halloween-Events zu Flyff! . Flyff Universe (Fly For Fun) is a cross-platform fantasy web 3D MMORPG published by Wemade Connect .
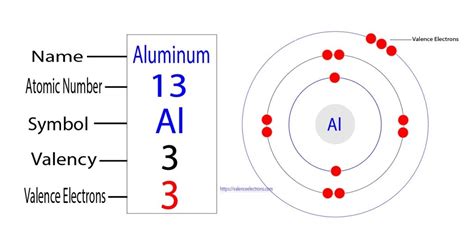
number of valence electrons in aluminum,After the electron configuration, the last shell of the aluminum atom has three electrons. In this case, the valency of aluminum is 3. The elements that have 1, 2, or 3 electrons in the last shell donate the electrons in the last shell during bond formation. The elements that form bonds by donating . Tingnan ang higit paThe second element in group-13 is aluminum. The valence electron is the total number of electrons in the last orbit. The total number of electrons in the last shell after the electron configuration of aluminumis called the valence electrons of aluminum(Al). . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some . Tingnan ang higit pa
number of valence electrons in aluminum How to Find the Valence Electrons for Aluminum (Al)?The valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to . Tingnan ang higit paAluminum participates in the formation of bonds through its valence electrons. We know that the valence electrons in aluminum . Tingnan ang higit pa

Mar 23, 2023
number of valence electrons in aluminum You may assume the valences of the chemical elements—the number of .

Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; . Electron Configuration of Aluminum. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Since aluminum's .
Answer: Aluminum has 3 valence electrons. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Aluminum .
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . An aluminum atom has three valence electrons. Do you think it will lose three electrons or gain five electrons to obtain an octet in its outermost electron shell? .This pattern allows us to quickly identify the number of valence electrons for main group elements on the periodic table, even if we don't have Bohr models to reference. The .
The atomic number of aluminum is 13. That is, the number of electrons in aluminum is thirteen. Therefore, the aluminum atom will have two electrons in the first shell, eight in the 2nd orbit, .
The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. In groups 1–12, the group number matches the number of valence electrons; in groups 13–18, the units digit of the group number matches the number of valence electrons. . Copper, aluminium .
Answer: Aluminum has 3 valence electrons. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Aluminum (Al), with an atomic number of 13, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1. In this configuration, the outermost shell is the third shell (3s 2 3p 1 ), which .
Aluminum is the 13th element of the periodic table so its atomic number is 13. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, an aluminum atom has thirteen protons and thirteen electrons. The number of neutrons in an atom can be determined by the difference between the . The electron configuration of aluminum is [ Ne] 3s 2 3p 1. In the above electron configuration, the highest energy level (3) is marked with green color. The 3 rd energy level contains 3s and 3p subshells. There are 2 electrons in the 3s subshell and 1 electron in the 3p subshell. So aluminum has a total of 2 + 1 = 3 valence electrons.Aluminum is the 13th element in the periodic table and has a symbol of Al and atomic number of 13. It has an atomic weight of 26.98154 and a mass number of 27. Aluminum has thirteen protons and fourteen neutrons in its nucleus, and thirteen electrons in three shells. It is located in group thirteen, period three and block p of the periodic .Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3 energy level which is the highest occupied energy level for magnesium. This corresponds to the 2+ 2 + charge formed when magnesium forms an ion.How to Find the Valence Electrons for Aluminum (Al)?Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .
number of valence electrons in aluminum|How to Find the Valence Electrons for Aluminum (Al)?
PH0 · What is the number of valence electrons in aluminum?
PH1 · Valences of the Chemical Elements
PH2 · Valence electrons (video)
PH3 · Valence Electrons Chart for All Elements
PH4 · How to Find the Valence Electrons for Aluminum (Al)?
PH5 · How many valence electrons does aluminum have?
PH6 · How Many Valence Electrons Does Aluminum (Al) Have?
PH7 · Determine valence electrons using the periodic table
PH8 · Chemistry of Aluminum (Z=13)
PH9 · 3.1: Valence Electrons
PH10 · 10.6: Valence Electrons